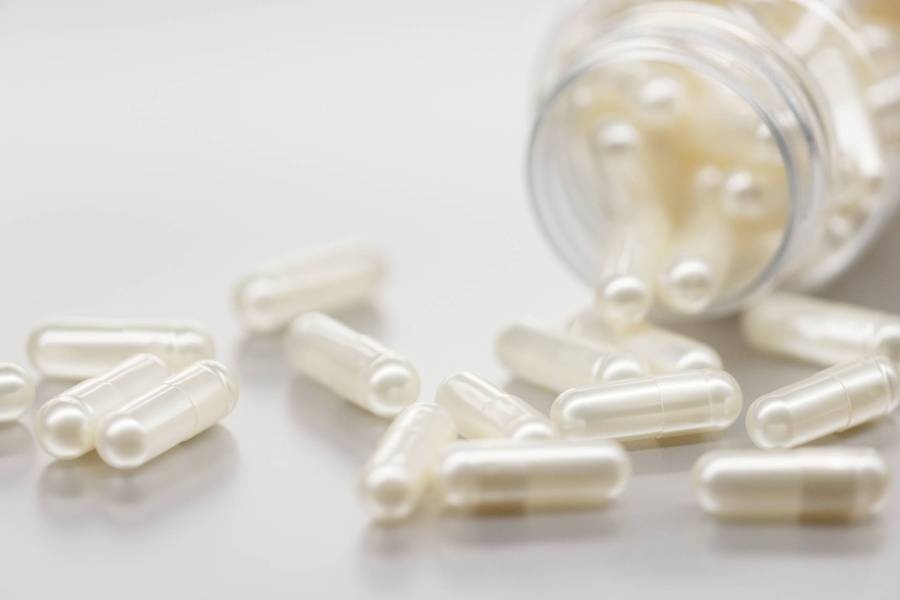
India's ACG pumps $200M into its first US empty-capsule production facility

ACG, a supplier and service provider for drug manufacturers, is pumping $200 million into what will be the India-based company’s first empty-capsule production facility in the U.S.
An initial $100 million will go toward developing a hard-shell capsule site in Atlanta, with the remaining half slated to be spent on expanding capacity and capabilities in the region, the company said in an Oct. 20 press release.
The project, when completed by early 2027, will be able to churn out gelatin and vegetarian hard-shell capsules and create more than 200 jobs, according to the company.
This facility strengthens our ties with customers across the region—bringing us closer to them, enabling faster lead times, higher-quality service, and a more resilient, de-risked supply chain,” Karan Singh, ACG managing director, said in the release. “Just as importantly, it lets us respond more quickly and co-develop new innovations through tighter R&D partnerships.”
The company has had a presence in the U.S. for more than a quarter-century, with its North American headquarters in Piscataway, New Jersey. The firm also operates a liquid-fill capsule facility in Chadds Ford, Pennsylvania, and several warehouses and sales teams across the U.S.
The U.S. project comes less than a year after ACG announced it would significantly expand its operations in Asia by building a new, 1.89 million-square-foot capsule production plant in Rayong on Thailand’s east coast. The facility—bigger than 32 football fields—will be able to produce about 20 billion hard gelatin capsules annually, the company said at the time.
That facility is expected to employ about 250 workers when completed.
Founded in Mumbai, India, in 1961, ACG has spent more than six decades expanding its global footprint and touts itself as having a presence in just abut every aspect of solid dosage production, ranging from capsule production to protective packaging.

 Go Back
Go Back