Could simple fluid bed modifications speed up your pellet layering?

Why high-dose substrate layering strains Wurster systems.
During Wurster coating, pellets cycle through a vertical spray column, where coating solution is applied layer by layer. Through fluidisation airflow, particles move between the spray zone and the surrounding drying zone.
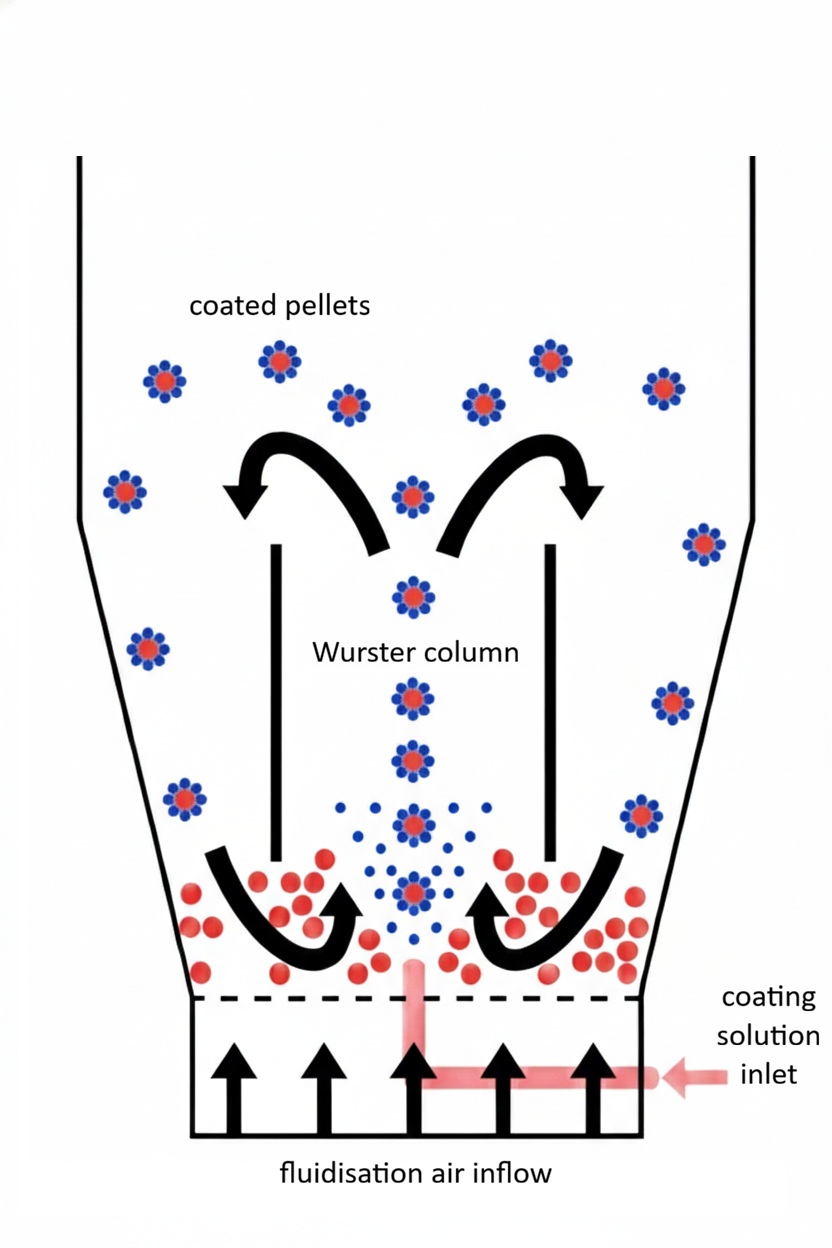
With each successive layer that’s sprayed and dried, solvent evaporation becomes the rate limiting step. Increasing coating concentration to reduce solvent load raises viscosity and nozzle clogging risk2, 3, while lower concentrations increase overages and extend drying times.
Studies using PEPT and CFD-DEM show that particles spend approximately 12–29% of the cycle time in the Wurster tube4. Since the rest of the cycle involves transport and heat/mass transfer in a separate zone, the spray-dry cadence is slow relative to rotor systems.
In rotor-based tangential spraying, wetting and drying overlap more.
Many modern fluid beds designed for Wurster coating can also accommodate rotor attachments, enabling tangential spraying. Rotor-based systems fundamentally change particle experience. Due to upward airflow, centrifugation and gravity, particles move in a helical or rope-like path around the chamber5.
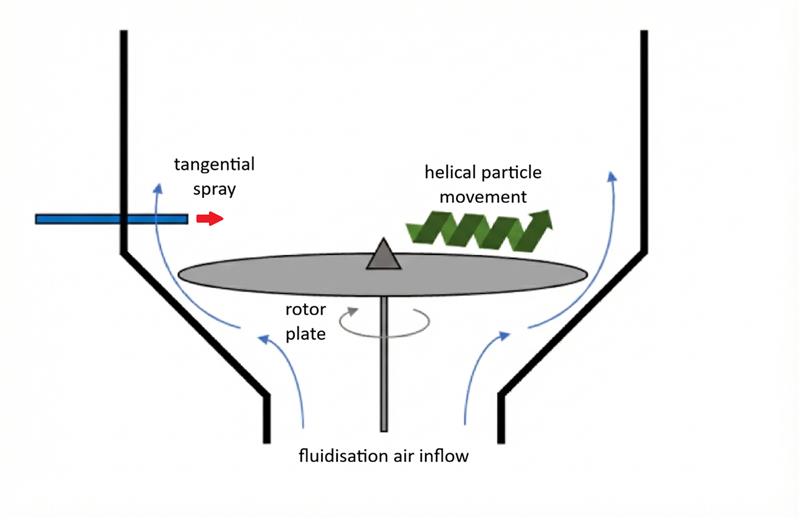
Shear forces and collisions mix pellets and spread droplets, so more surface area is exposed for drying, and localised solvent accumulation is minimised.
All pellets move simultaneously, unlike intermittent exposure in a Wurster column. So layering, drying, and mixing proceed concurrently.
Rotary fluid bed variables must act in synchronicity.
Spray rate, atomisation pressure, and rotor speed predominantly affect particle size distribution (PSD) and growth5. High spray rates at low atomisation pressures produce larger, slower-to-evaporate droplets, yielding larger agglomerates and a broader PSD5. Conversely, finer droplets from higher pressure distribute more uniformly, narrowing PSD.
Rotor speed introduces a moisture-dependent mechanical effect. At low spray rates, high speeds break weak, dry agglomerates, keeping particle size small. At high spray rates, high speeds enhance the compaction and stability of wet, plastically deformable particles, increasing their size5.
The implication is clear: rotor systems tolerate high deposition rates, but pushing either rotor speed or spray rate independently can destabilise the process.
Dry powder layering shifts spraying from carrier to adhesive.
Even in rotor-based tangential spraying, solvent evaporation still governs how aggressively deposition rates can be pushed. Dry powder layering changes this by redefining the role of the liquid phase – from carrier to adhesive.
Simultaneously spraying binder solution with API powder minimises solution sprayed on each particle, and shortens the drying time required per cycle.
Compared with tangential spraying of solutions, powder layering can reduce solvent use (particularly aqueous) by up to 80%, and cut process times by almost 50%6.
This helped a global CDMO cut process time by 60%.
Recently, one of our customers (a CDMO serving regulated markets) was developing a Wurster process to prepare pellets for Lansoprazole Capsules.
To prevent nozzle clogging, the spray concentration was limited to 25%. However, a low concentration required lots of solvent for the same amount of drug loading. Their material overages were capped at 5% above theoretical requirements. So, simply using more solution was out of the question.
With process engineers at ACG Laboratories, they switched to rotor-based dry powder layering.
The rotor was inserted in the existing machine, along with a powder-feeding attachment. This allowed rapid mass build up with minor modifications and minimal investment.
Together, we achieved:
- 60% reduction in process time (from 12 to nearly 5 hours)
- 70% reduction in solvent consumption
- 100% reduction in material overages (from 5% to 0%)
- 100% pellet coating efficiency
Conclusion.
Wurster systems excel at applying uniform functional films. But when the objective shifts to high-dose substrate layering, the process becomes constrained by solvent evaporation and intermittent spray exposure, not just machine settings.
Rotor-based tangential spraying changes particle behaviour, allowing wetting, mixing, and drying to proceed concurrently rather than sequentially. Dry powder layering goes one step further by reducing the liquid phase from a carrier to an adhesive role, sharply lowering solvent demand and process time.
Matching the deposition mechanism to the loading objective can unlock large gains; sometimes with only minor equipment modifications.
References:
- Suhrenbrock et al. (2012). Pellet layering: scale-up considerations using different kinds of processing equipment. Drug Development and Industrial Pharmacy Volume 38, 2012 - Issue 12.
https://www.tandfonline.com/doi/10.3109/03639045.2011.653815?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed - Alkadhem et al. (2025). Engineering Catalysts at the Multiscale: Past, Present, and Future of Catalyst Manufacturing and Shaping Processes. ChemCatChem 2025, 0, e01109.
https://d-nb.info/1380103029/34 - Mueller et al. (2023). Evaluation of a Three-Fluid Nozzle Spraying Process for Facilitating Spray Drying of Hydrophilic Polymers for the Creation of Amorphous Solid Dispersions. Pharmaceutics 2023, 15(11), 2542.
https://www.mdpi.com/1999-4923/15/11/2542 - Li, Liang. (2015). Particle motion, coating and drying in Wurster fluidized beds - An experimental and discrete element modeling study. Department of Chemistry and Chemical Engineering Chalmers University of Technology.
https://publications.lib.chalmers.se/records/fulltext/224608/224608.pdf - Langner et al. (2023). Statistical Investigation of Rotary Fluidized Bed Agglomeration Process with Tangential Spray and In-Line Particle Size Measurement for PAT Process Control. Processes 2023, 11(4), 1066.
https://www.mdpi.com/2227-9717/11/4/1066 - Foppoli et al. (2020). Evaluation of powder-layering vs. spray-coating techniques in the manufacturing of a swellable/erodible pulsatile delivery system. Drug Dev Ind Pharm 2020 Aug;46(8):1230-1237.
https://pubmed.ncbi.nlm.nih.gov/32597251/

 Go Back
Go Back





